The DARGER™ platform
Unlocking the full potential of oligonucleotides
The DARGER™ Platform (Dual-functional Antisense RNA for Gene Expression Regulation) is a next-generation RNA discovery engine that combines super-active siRNA for potent gene silencing with ACT-UP1, a novel ASO-based technology for gene upregulation. These approaches enable broad therapeutic potential across diverse disease areas.
Unique upregulation
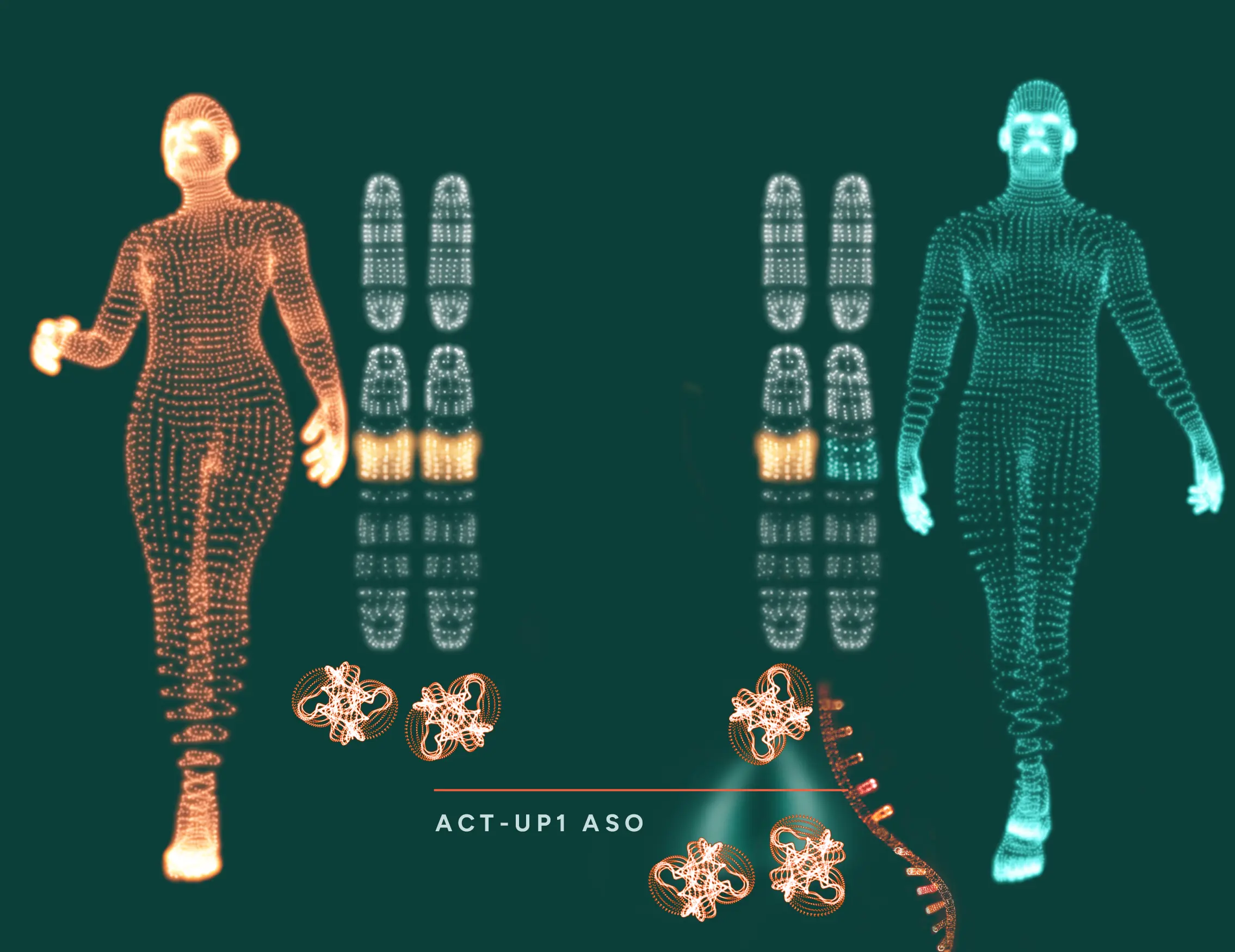
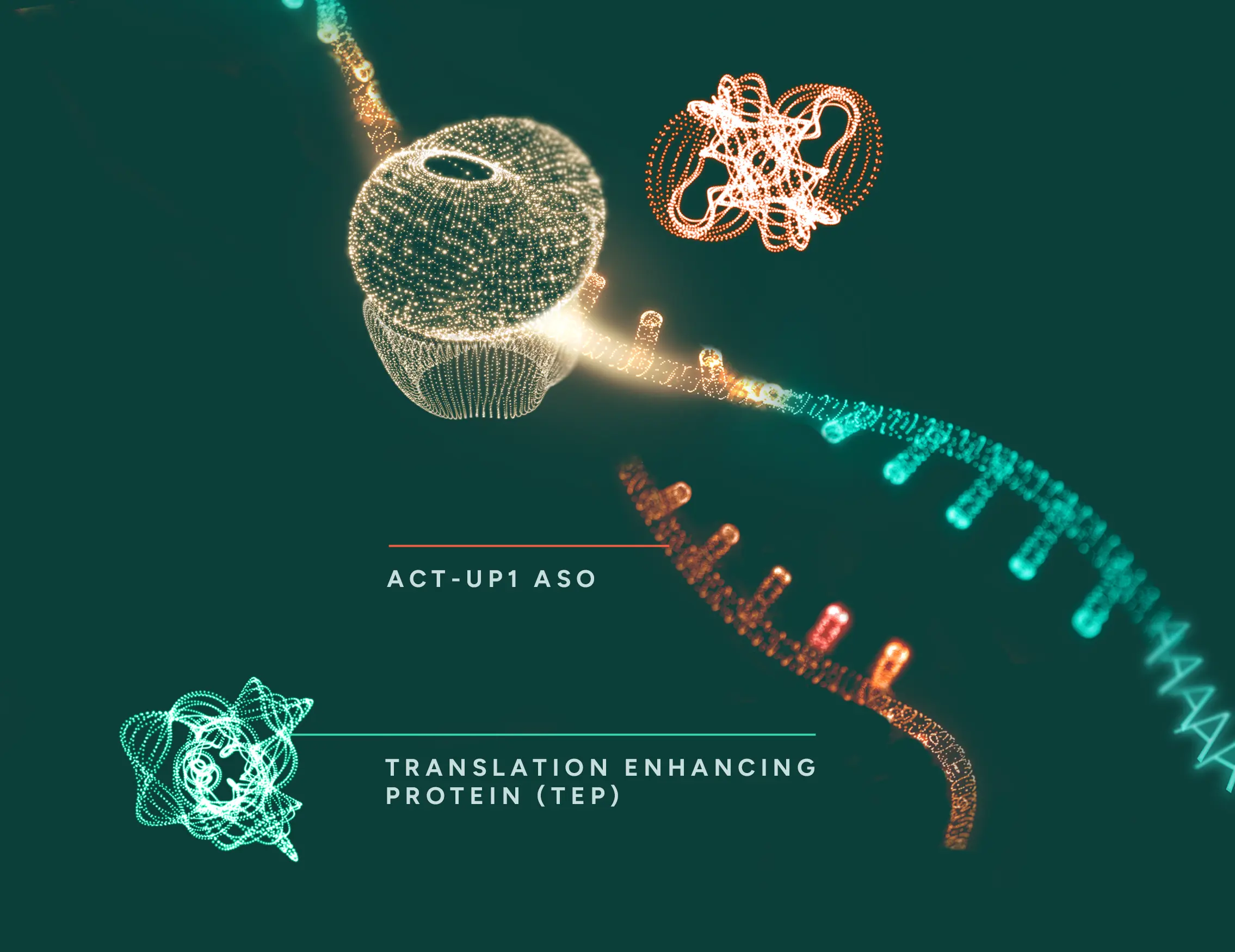
ACT-UP1 represents a novel approach to increasing specific protein levels using ASOs to enhance translation.
The ASO design follows well-established principles to ensure efficient cellular uptake and target specificity. What differentiates ACT-UP1 is its proprietary motif, which recruits cellular translation-enhancing proteins to the bound mRNA, leading to increased protein expression. Unlike other approaches, ACT-UP1 has the potential to upregulate any gene of interest without relying on elements present in the target mRNA.
Targeting the root cause
ACT-UP1 is applicable to a range of genetic disorders, including:
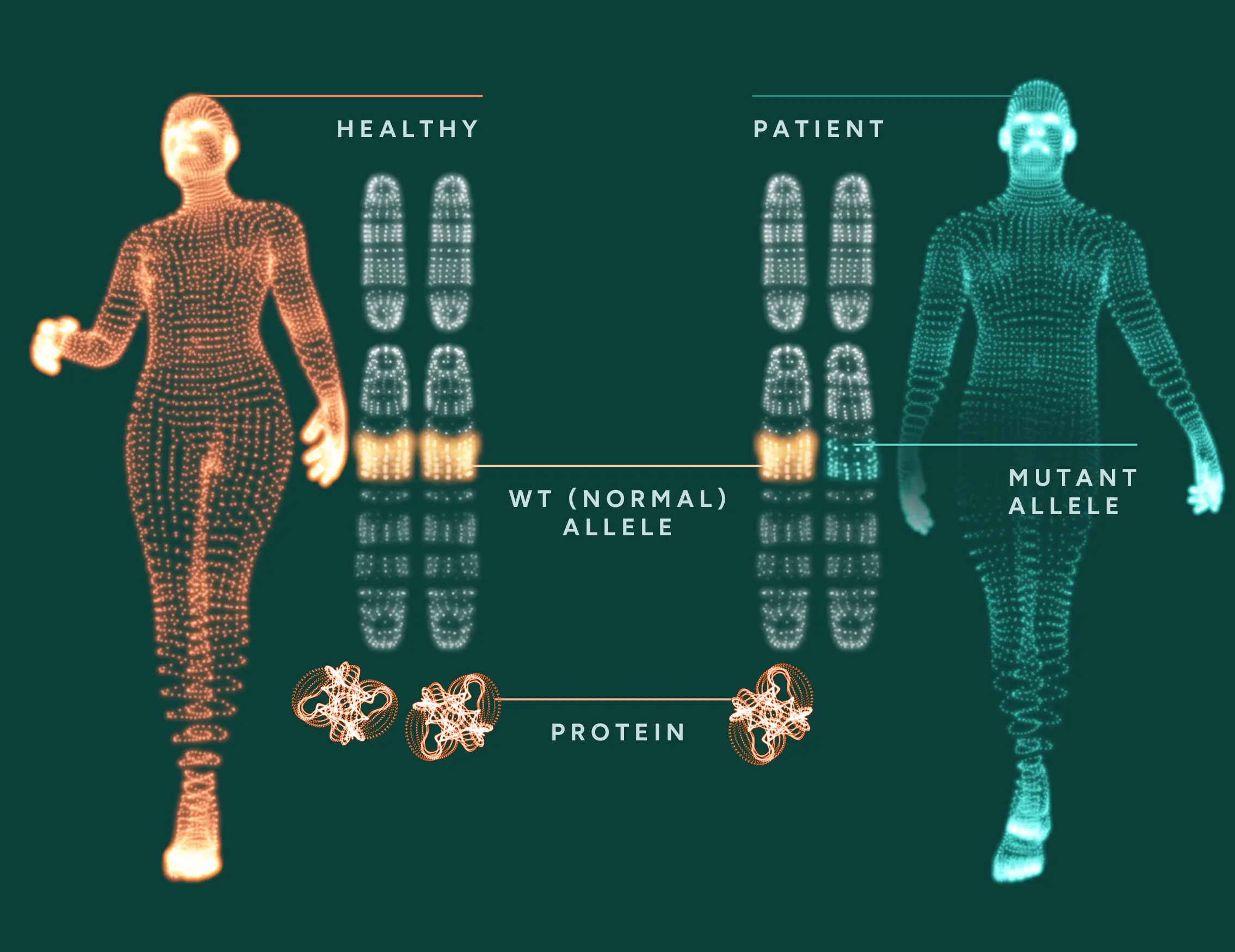
- Haploinsufficiency – conditions in which an individual has only one functional copy of a gene, while the other allele carries a mutation that fails to produce a functional protein. The single functional copy does not generate sufficient gene product (typically a protein) to maintain normal physiological function. This reduced output can lead to disease or abnormal phenotypes.
- Disorders caused by insufficient protein levels – where protein production is not enough for proper cellular function.
In both cases, increasing the amount of functional protein addresses the underlying molecular deficiency and offers a therapeutic strategy.
Translation efficiency is rate-limited at initiation
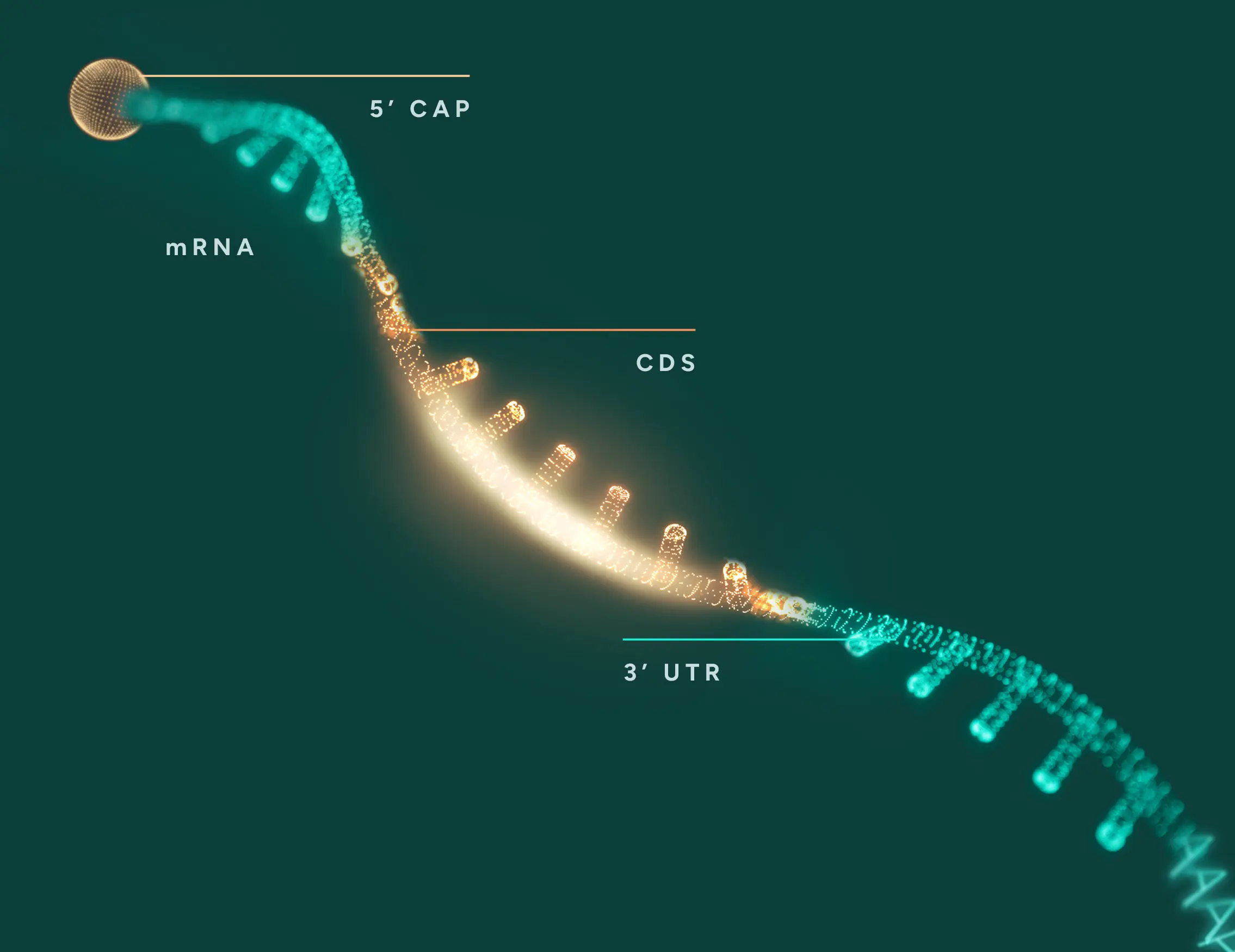
Protein synthesis, or translation, is the process of producing protein from mRNA template.
This process is typically rate-limited by the translation initiation step, which is the first and most regulated phase. During initiation, the translation machinery including ribosome assembles on the mRNA and begins decoding at the correct start codon—critical for ensuring the accuracy of the resulting protein. Enhancing this step can significantly increase protein output.
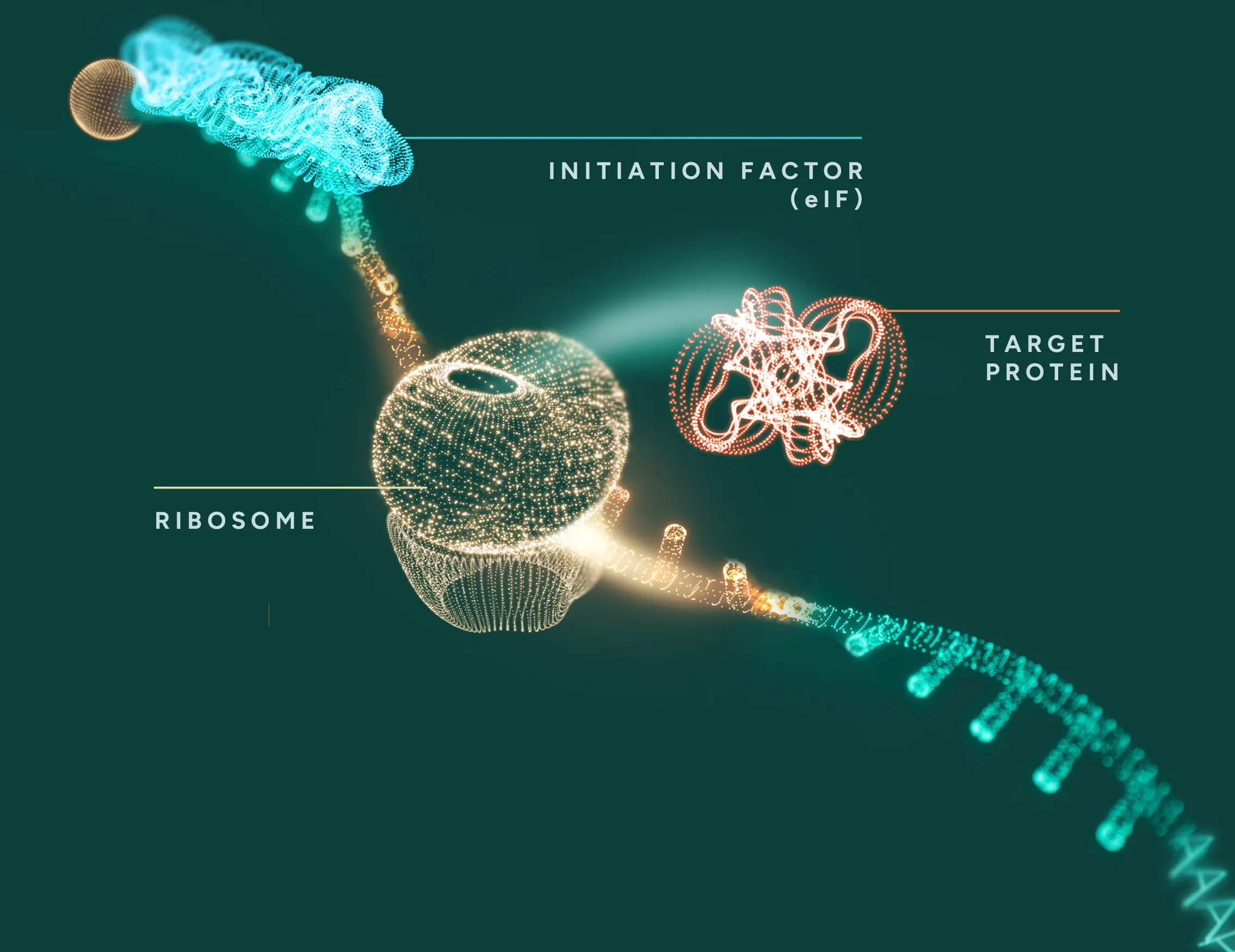
ACT-UP1 binds and recruits
The ACT-UP1 ASO is engineered to bind specifically to the target mRNA that encodes a protein of therapeutic interest.
Once bound, the ACT-UP1 proprietary ASO recruits cellular proteins that are required for efficient initiation of translation, effectively priming the mRNA for enhanced protein synthesis.
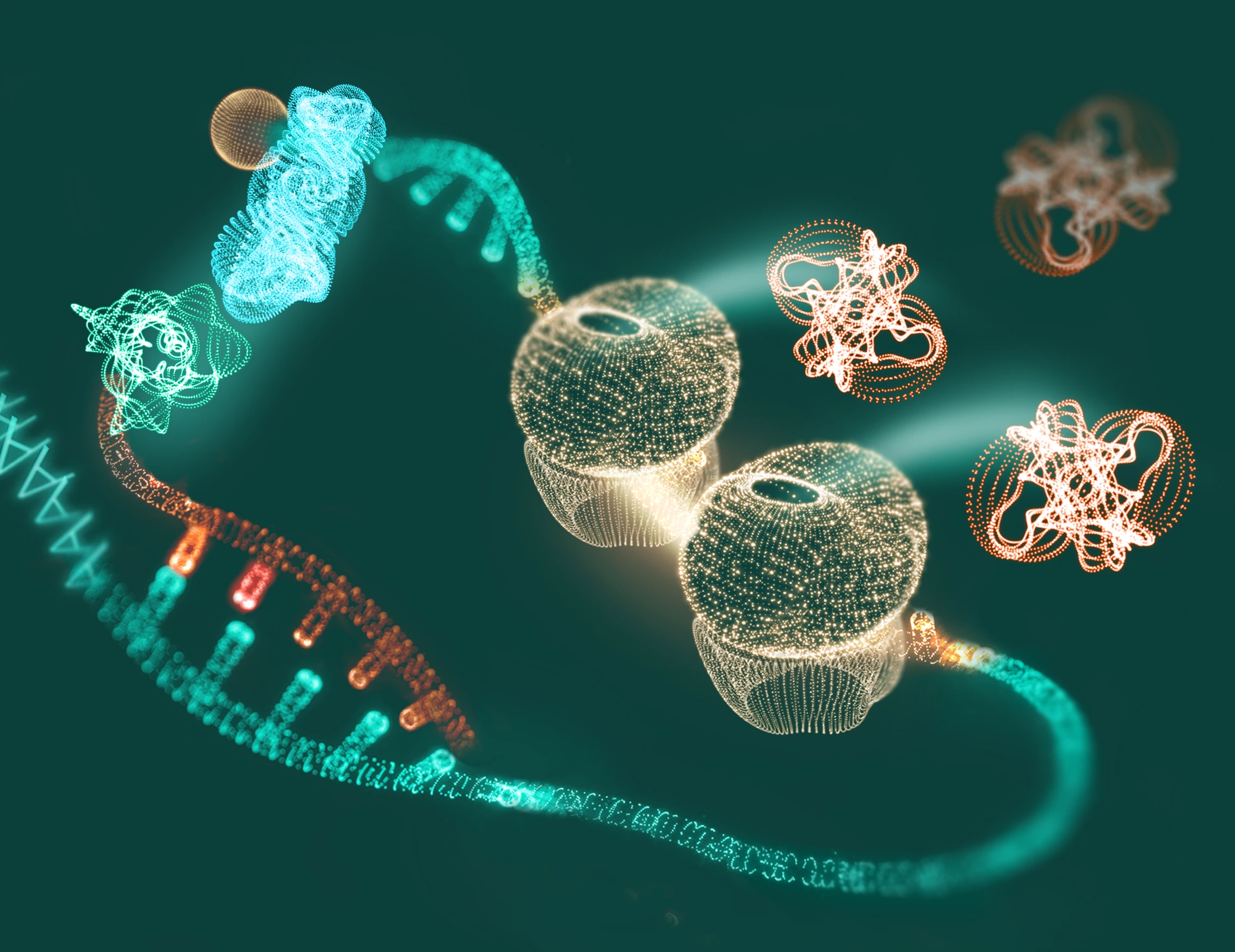
Amplifying protein production
The recruited proteins boost translation efficiency, resulting in increased production of functional protein.
This is achieved by leveraging the cell’s own translational machinery—without the need to introduce exogenous proteins or modify the genetic code.
Super-active downregulation
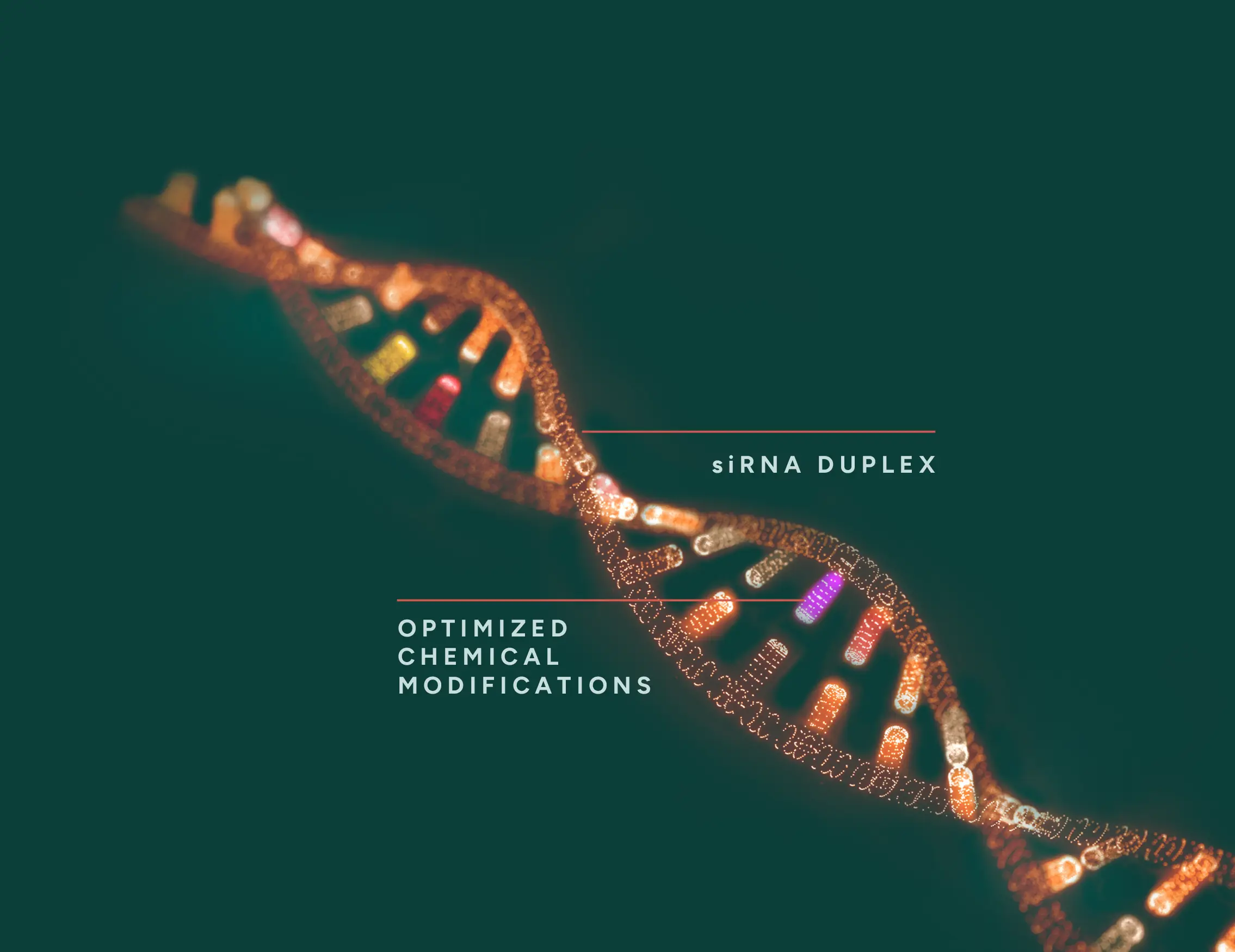
Arnatar’s siRNA platform delivers faster and more potent gene silencing due to optimized chemical modifications.
Based on decades of experience in antisense technology and RNA biology, Arnatar enhances its siRNA molecules with precise chemical changes to improve potency, specificity, stability and safety. These modifications are illustrated in the colored regions of step 2 and have the following advantage:
Efficient RISC loading
Our siRNAs are designed for superior loading into the RISC protein complex, resulting in greater potency at lower doses.
Faster strand unwinding
The optimized chemistry also makes it easier for the siRNA strands to unwind, which enables a faster onset of action compared to other platforms.
AI-powered oligo design
We leverage a proprietary AI-based algorithm that utilizes decades of deep domain know-how of RNA biology, antisense technology, disease mechanisms, and chemical modifications to rationally design better oligonucleotide therapeutics. This approach ensures an efficient lead identification process.
Proprietary GalNAc
Arnatar’s proprietary GalNAc conjugation technology is a next-generation liver-targeting platform designed to enhance the delivery of oligonucleotide therapeutics. It achieves high-delivery efficiency, ensuring optimal uptake by hepatocytes while maintaining an excellent safety profile.
Unlike conventional GalNAc designs that often require over 20 synthetic steps, Arnatar’s streamlined chemistry enables GalNAc synthesis in just 7 steps, drastically reducing production time and cost. This simplified synthesis not only accelerates development timelines but also improves scalability and manufacturing consistency.
By combining superior delivery, efficient synthesis, and proven safety, Arnatar’s GalNAc technology offers a powerful and differentiated approach for advancing RNA-based therapies targeting liver and systemic diseases.


